Airway management is an essential medical procedure that can be carried out through invasive and non-invasive procedures. Practices like CPR, chest compressions and the use of a bag valve mask are all examples of non-invasive airway management procedures, intended to help someone unable to breathe without the use of implanted medical devices or intubation. More serious airway management emergencies require more advanced airway management procedures, with devices specially made for the oropharyngeal airway, the nasopharyngeal airway, and the endotracheal airway with intubation.
Molded Devices, Inc. offers a true single-source vendor option for medical device companies, offering advanced product development and engineering services from ideation to prototype, to final production. We routinely work with medical device companies to help develop advanced oropharyngeal airway (OPA), nasopharyngeal airway (NPA), and endotracheal airway (ETA) devices, engineered from difficult materials and complex designs.
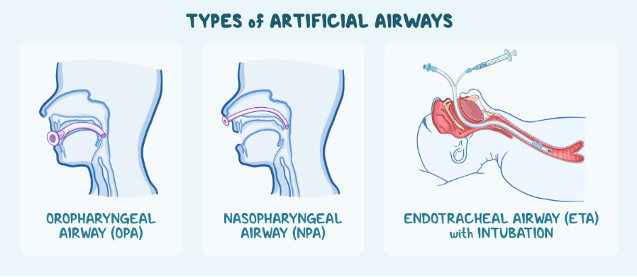
Oropharyngeal Airway (OPA) Device
This medical device is designed for unresponsive individuals and is inserted through the mouth and throat. These devices work to maintain and open a breathing hole by preventing the tongue from covering the epiglottis, or a small flap in the back of the throat.
Nasopharyngeal Airway (NPA) Device
Similar to OPAs, NPA devices maintain an opening in the back of the throat, through the insertion of a device in the nasal cavity. These devices are often better tolerated by conscious patients, and therefore may be considered if the patient is responsive and refuses the OPA device. These can also be helpful in a scenario where the patient’s jaw may be clenched.


Recent Comments